
Subscribe | The Record Archive | Contacts | bcbsm.com
|
June 2024
Here’s how medical residents can join our networkMedical residents interested in joining our network can submit their Blue Cross Blue Shield of Michigan or Blue Care Network provider enrollment applications up to 60 days before they complete their training. It’s important to apply within the required time frame. If medical residents apply more than 60 days before the completion of residency training, we’ll deny the application and residents will have to reapply. The CAQH Provider Data Portal,** formally known as CAQH ProView, application must be completed to begin the credentialing process with Blue Cross and BCN. To keep CAQH information current, complete the re-attestation every 120 days and update the Authorize section. Visit the CAQH Provider Data Portal** for more information on application requirements. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
New mini modules available to help you navigate Blue Cross behavioral health provider portalThe provider training team would like to introduce two new mini modules that will help with some common issues our behavioral health providers sometimes experience within the Blue Cross Blue Shield of Michigan behavioral health provider portal:
These mini modules take less than four minutes to complete and give tips on how you can resolve issues within the portal. Whether you are receiving an error at sign on or when attempting to search an authorization, these modules can show you how to resolve issues to get you the outcome you are looking for. You can find these mini modules on the provider training site by searching “behavioral health” or “mini” in the search box on the upper right corner of the page. To access the training site, follow these steps:
If you’re a new training site user, complete the one-time registration by entering your role and creating a password. This allows you to access the training site outside of the provider portal if needed. If you need assistance navigating the provider training site, email ProviderTraining@bcbsm.com. **Blue Cross Blue Shield of Michigan doesn’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Use modifiers JW and JZ when billing Part B medical benefit drug claimsTo receive timely and appropriate payment of Part B claims for Medicare Plus Blue℠ and BCN Advantage℠ members, health care providers, facilities and suppliers must include the JW or JZ modifier when billing for single-dose vials or other single-use packages of Part B drugs. This doesn’t apply to multiuse vials or other multiuse packages. For claims submitted on or after Oct. 1, 2023, the Centers for Medicare & Medicaid Services requires health plans to return claims without processing them when claim lines don’t include the appropriate modifiers. The claims must then be resubmitted with the appropriate modifiers. This applies to all providers, facilities and suppliers who buy and bill separately payable single-container drugs under Medicare Part B. Here's how to use these HCPCS Level II modifiers:
Example: A single-use vial that is labeled to contain 100 units of a drug has 95 units administered to the member and five units discarded. The 95-unit dose is billed on Line 1, while the discarded five units are billed on Line 2 using the JW modifier. Both line items are processed for payment. Providers must record the discarded amounts of drugs and biologicals in the member’s medical record.
Here’s what you need to include on these claims:
For additional information, see the CMS Billing and Coding: JW and JZ Modifier Billing Guidelines** page on cms.gov.** ** Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
2024 HCPCS 1st-quarter update: New codes added and deletedThe Centers for Medicare & Medicaid Services has added several new codes as part of its quarterly Health Care Procedure Coding System updates. The codes, effective dates and Blue Cross Blue Shield of Michigan’s coverage decisions are below. Other medical service
Surgery/skin substitute
Medicine/alcohol and drug abuse treatment services
Durable medical equipment
Prosthetic procedures
Orthotic procedures
Medical/surgical supplies
Injections/chemotherapy
Injections/behavioral health
Injections
Outpatient prospective payment system/surgery
Deleted codes
None of the information included in this article is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
HCPCS replacement codes established, effective April 1, 2024C9166 replaces C9399 when billing for Cosentyx® (secukinumab) Effective April 1, 2024, the Centers for Medicare & Medicaid Services, or CMS, has established a permanent procedure code for the specialty medical drug Cosentyx (secukinumab). Services can continue to be reported with C9399 through March 31, 2024. All services performed on and after April 1, 2024, must be reported with C9166. C9168 replaces J3590 when billing for Omvoh™ (mirikizumab-mrkz) Effective April 1, 2024, CMS has established a permanent procedure code for the specialty medical drug Omvoh (mirikizumab-mrkz). Services can continue to be reported with J3590 through March 31, 2024. All services performed on and after April 1, 2024, must be reported with C9168. J0177 replaces C9161, J3490, J3590 and J9999 when billing for Eylea® HD (aflibercept) Effective April 1, 2024, CMS has established a permanent procedure code for the specialty medical drug Eylea HD (aflibercept). Services can continue to be reported with C9161, J3490, J3590 and J9999 through March 31, 2024. All services performed on and after April 1, 2024, must be reported with J0177. J0589 replaces C9399, J3490, J3590 and J9999 when billing for Daxxify® (daxibotulinumtoxinA-lanm) Services can continue to be reported with C9399, J3490, J3590 and J9999 through March 31, 2024. All services performed on and after April 1, 2024, must be reported with J0589. J1323 replaces C9165 when billing for Elrexfio™ (elranatamab-bcmm) Effective April 1, 2024, CMS has established a permanent procedure code for Elrexfio (elranatamab-bcmm). Services can continue to be reported with C9165 through March 31, 2024. All services performed on and after April 1, 2024, must be reported with J1323. J2782 replaces C9162, C9399, J3490, J3590 and J9999 when for billing IZERVAY™ (avacincaptad pegol) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug IZERVAY (avacincaptad pegol). All services through March 31, 2024, will continue to be reported with C9162, C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2024, must be reported with J2782. Prior authorization is required through the Medical Benefit Drug program for J2782 for all groups unless they are opted out of the program. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. For groups that have opted out of the prior authorization program, this code is covered for the FDA-approved indications. J3055 replaces C9163 when billing for TALVEY™ (talquetamab-tgvs) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug TALVEY (talquetamab-tgvs). All services through March 31, 2024, will continue to be reported with C9163. All services performed on and after April 1, 2024, must be reported with J3055. J7165 replaces C9159 when billing for BALFAXAR® (prothrombin complex concentrate, human-lans) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug BALFAXAR (prothrombin complex concentrate, human-lans). All services through March 31, 2024, will continue to be reported with C9159. All services performed on and after April 1, 2024, must be reported with J7165. J7354 replaces C9164 when billing for YCANTH™ (cantharidin) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug YCANTH (cantharidin). All services through March 31, 2024, will continue to be reported with C9164. All services performed on and after April 1, 2024, must be reported with J7354. J9376 replaces C9399, J3490, J3590 and J9999 when billing for VEOPOZ™ (pozelimab-bbfg) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug VEOPOZ (pozelimab-bbfg). All services through March 31, 2024, will continue to be reported with C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2024, must be reported with J9376. Prior authorization is required through the Medical Benefit Drug program for J9376 for all groups unless they are opted out of the program. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. For groups that have opted out of the prior authorization program, this code is covered for the FDA-approved indications. Q5133 replaces C9399, J3490, J3590 and J9999 when billing for TOFIDENCE™ (tocilizumab-bavi) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug TOFIDENCE (tocilizumab-bavi). All services through March 31, 2024, will continue to be reported with C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2024, must be reported with Q5133. Prior authorization is required through the Medical Benefit Drug program for Q5133 for all groups unless they are opted out of the program. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. For groups that have opted out of the prior authorization program, this code is covered for the FDA-approved indications. Q5134 replaces C9399, J3490, J3590 and J9999 when billing for TYRUKO® (natalizumab-sztn) Effective April 1, 2024, CMS has established a new procedure code for the specialty medical drug TYRUKO (natalizumab-sztn). All services through March 31, 2024, will continue to be reported with code C9399, J3490, J3590 and J9999. All services performed on and after April 1, 2024, must be reported with Q5134. Prior authorization is required through the Medical Benefit Drug program for Q5134 for all groups unless they are opted out of the program. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. For groups that have opted out of the prior authorization program, this code is covered for the FDA-approved indications.
Be sure to use the correct provider taxonomy code to avoid payment delaysWe use taxonomy codes to assist in the identification of a provider when they're using one NPI for several Blue Cross Blue Shield of Michigan provider IDs. Blue Cross and Blue Care Network claims payment systems don’t use every taxonomy code listed in the National Uniform Claim Committee, or NUCC, code set list. In the enrollment and credentialing process, if you submit a taxonomy code that we don’t use, we’ll change it to a higher-level taxonomy code. You must use the Blue Cross- and BCN-assigned taxonomy code during the billing process to avoid possible payment delays. This applies to both Blue Cross and BCN commercial and Medicare Advantage plans. Taxonomy codes designate your provider specialty. To find the provider taxonomy code you’re required to use when submitting electronic claims to Blue Cross and BCN, refer to these documents located on bcbsm.com: Here are some examples:
Billing chart: Blue Cross highlights medical, benefit policy changesYou’ll find the latest information about procedure codes and Blue Cross Blue Shield of Michigan billing guidelines in the following chart. This billing chart is organized numerically by procedure code. Newly approved procedures will appear under the New Payable Procedures heading. Procedures for which we have changed a billing guideline or added a new payable group will appear under Updates to Payable Procedures. Procedures for which we are clarifying our guidelines will appear under Policy Clarifications. New procedures that are not covered will appear under Experimental Procedures. We'll publish information about new Blue Cross groups or changes to group benefits under the Group Benefit Changes heading. For more detailed descriptions of the Blue Cross' policies for these procedures, check under the Commercial Policy tab in Benefit Explainer on Availity®. To access this online information:
2 .Click on Payer Spaces on the Availity menu bar. 3. Click on the BCBSM and BCN logo. 4. Click on Benefit Explainer on the Applications tab. 5. Click on the Commercial Policy tab. 6. Click on Topic. 7. Under Topic Criteria, click on the circle for Unique Identifier and click the drop-down arrow next to Choose Identifier Type, then click on HCPCS Code. 8. Enter the procedure code. 9. Click on Finish. 10. Click on Search.
None of the information included in this billing chart is intended to be legal advice and, as such, it remains the provider’s responsibility to ensure that all coding and documentation are done in accordance with all applicable state and federal laws and regulations.
Talk to Medicare Advantage members about how to maintain independence and confidenceTo help our Medicare Plus Blue℠ and BCN Advantage℠ members remain independent and feel confident as they age, we’ve asked them to talk to their health care providers about the following issues:
We’re encouraging our Medicare Advantage members to share their concerns with you like they would with a close friend. We’re suggesting they write down their concerns and read from the list or hand it to you so you can start the discussion. We also encourage you to discuss these issues with patients even if the patient doesn’t initiate the conversation. Many patients don’t ask questions about these topics because they forget or don’t know what to ask, they’re embarrassed or they assume they have to “live with it.” When you bring up these topics, it opens the door to a conversation that may not otherwise happen. It also helps your patients to know these are common issues and what types of questions they should ask going forward. We appreciate your efforts to make members as comfortable as possible when discussing sensitive issues.
We invite you to join PGIP as a physician organizationBlue Cross Blue Shield of Michigan will accept applications from new physician organizations for the Physician Group Incentive Program from June 1 through July 31, 2024. To request application materials, email valuepartnerships@bcbsm.com. About PGIP PGIP was developed with input from providers across Michigan to help improve the quality and efficiency of health care in the state. PGIP facilitates change through a wide range of initiatives, including our nationally recognized Patient-Centered Medical Home program. Through PGIP, we reward physician organizations for improving health care delivery to their attributed patient populations. Participating physicians are eligible for value-based reimbursement consideration as a result of program efforts. A PGIP physician organization consists of physicians participating in our PPO or Traditional network who work together to:
To learn more
Blue Cross, BCN to begin reimbursing E/M when billed with preventive serviceBlue Cross Blue Shield of Michigan and Blue Care Network will begin reimbursing for evaluation and management, or E/M, services at 50% of the allowed amount when billed on the same day as a preventive service. (See list below.) The preventive service will pay in full. This is a change from Blue Cross and BCN’s current policy that only pays for the preventive service. When two services are done on the same day, the modifier 25 must be billed with the E/M code or it won’t be paid. This reimbursement change will be effective for dates of service beginning June 1, 2024. If a denial occurs while the claim system is being updated, resubmit your claim after the update is complete. The update is expected to be completed in late June. Do not submit an appeal for dates of service after June 1. E/M codes reimbursed at 50%: *99201–*99205 Preventive codes: *99381–*99387
Save time by submitting only required information about acute inpatient medical, surgical admissionsWhen health care providers submit a prior authorization request for an acute inpatient medical-surgical admission, they can save time by submitting only the information that’s required for the request. Refer to the table below for more information.
TurningPoint Healthcare Solutions LLC is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network.
AllianceRx Walgreens to become exclusive specialty drug pharmacy for MESSA, starting July 1, 2024AllianceRx Walgreens Pharmacy will become the exclusive specialty drug network for the Michigan Education Special Services Association, known as MESSA, starting July 1, 2024. MESSA members who take a specialty drug have been directed to transfer their specialty medication to AllianceRx Walgreens. If you have patients who don’t currently fill their specialty drugs with AllianceRx Walgreens, you’ll need to transfer their prescriptions to AllianceRx Walgreens before July 1, 2024. If MESSA members fill their specialty medication through a different pharmacy, beginning July 1, 2024, they may be responsible for the full cost of the drug. Note: On Aug. 1, 2024, AllianceRx Walgreens Pharmacy will change its name to Walgreens Specialty Pharmacy.
Guidelines for using Autism diagnostic evaluation results formMembers can obtain an autism evaluation using one of the methods described in the document Obtaining an autism diagnostic evaluation and finding treatment. If you choose to record the results of the autism evaluation on the Autism diagnostic evaluation results form, follow these guidelines:
Note: We’re still receiving the older forms from approved autism evaluation centers. Faxing these forms to Blue Cross Blue Shield of Michigan and Blue Care Network — instead of giving them to the member or to the member’s parent or guardian — can delay the members from getting the treatment they need.
We’ve updated the document Blue Cross Behavioral Health: Frequently asked questions for providers to include this information. Providers can access that document on our ereferrals.bcbsm.com website, on these pages:
Cancer screening resources to share with patientsThis is part of an ongoing series of articles focusing on the tools and resources available to help FEP® members manage their health. In the United States, 1 in 2 men and 1 in 3 women will be diagnosed with cancer in their lifetimes, according to the American Cancer Society. Regular screening increases the chances of detecting certain cancers early before the cancer spreads. To promote prevention and early detection of cancer, the American Cancer Society developed Prevention and Early Detection Guidelines.** Its website offers information on how cancer screening guidelines are created, and has clinician and patient-friendly versions on current screening guidelines. Here are some additional resources to share with patients about cancer screening:
The Blue Cross and Blue Shield Federal Employee Program® covers screenings for breast cancer, cervical cancer, colon cancer and prostate cancer with no out-of-pocket costs when a member is seen by a Preferred provider. Health care providers and members can call Customer Service at 1-800-482-3600 or go to fepblue.org if they have questions about FEP benefits. **Blue Cross Blue Shield of Michigan doesn’t own or control this website.
Webinars for physicians, coders focus on risk adjustment, codingWe’re offering webinars about documentation and coding of common challenging diagnoses. These live, lunchtime educational sessions will also include an opportunity to ask questions. Below is our schedule and the tentative topics for the sessions. All sessions start at noon Eastern time and generally last for 30 minutes. Register for the session that best works with your schedule on the provider training website.
Provider training website access Provider portal users with an Availity® Essentials account can access the provider training website by logging in to availity.com,** clicking on Payer Space in the top menu bar and then clicking on the BCBSM and BCN logo. Then click on the Applications tab, scroll down to the Provider Training Site tile and click on it. You can also directly access the training website if you don’t have a provider portal account: Provider training website. After logging in to the provider training website, look in Event Calendar to sign up for your desired session. You can also quickly search for all the sessions with the keyword “lunchtime" and then look under the results for Events. You can listen to the previously recorded sessions too. Check out the following:
Questions?
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
We’ve enhanced the Authorizations & Referrals tool in Availity EssentialsIn May 2024, we made an enhancement to the Authorizations & Referrals tool in our provider portal, Availity® Essentials. This enhancement makes it easier for health care providers to determine if a Carelon-managed procedure code requires prior authorization for an in-state or out-of-state Blue Cross Blue Shield of Michigan commercial member. Now, when you use the Authorizations & Referrals tool to look up the prior authorization requirement for a Carelon-managed procedure code, you’ll be directed to Carelon if prior authorization is required for the service for that specific member. If prior authorization isn’t required, you’ll see a message to that effect. To access the Authorizations & Referrals tool:
For instructions on how to use the Authorizations & Referrals tool, see the document titled Determining prior authorization requirements for members. You can also access this document by clicking the Determine prior authorization requirements for members tile on the left side of any page on our ereferrals.bcbsm.com website. **Blue Cross Blue Shield of Michigan doesn’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services. Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
No application necessary for certain specialties to join BlueHPNThe Blue High Performance Network℠, also called BlueHPN℠, no longer requires the following specialties in the Detroit market to apply for the network to keep their active status:
This is in addition to behavioral health specialties, which we reported in the January Record require no application to keep their active status. The effective date of participation all the specialties listed above went into effect Jan. 1, 2024. To support adequate access to the specialties listed above, Blue Cross Blue Shield of Michigan will use the TRUST PPO or Traditional network to support BlueHPN to ensure inclusivity to members who need these services. By removing the application process for these specialties, it allows BlueHPN members to choose from a broader range of in-network providers for these services, while increasing access to care. Providers who are part of the BlueHPN must at a minimum:
If you have questions, email the BlueHPN team at BlueHPN@bcbsm.com.
Change to prior authorization process for Blue Cross commercial members in Michigan whose plans have local provider networksBlue Cross Blue Shield of Michigan currently has one Blue Cross commercial plan in Michigan that has a local provider network. That plan is the Blue High Performance Network℠, or BlueHPN℠. Because this plan has a local provider network, providers must be in that network for services to be reimbursable. Starting June 27, 2024, we’ll update the prior authorization process for members with BlueHPN plans to ensure that providers are in network. We’ll also do this for any Blue Cross commercial plans with local provider networks that are added in the future for members in Michigan. Here’s what we’ll do on and after June 27 when we receive prior authorization requests for members with these plans:
Reminders As always, it’s essential that providers check each member’s eligibility and benefits prior to performing services. Providers are responsible for identifying the need for prior authorization through our provider portal, Benefit Explainer or Provider Inquiry and for obtaining prior authorization for services, as needed.
New webpage provides Medicare Advantage prior authorization clinical review criteria in one convenient locationBlue Cross Blue Shield of Michigan and Blue Care Network’s Medicare Advantage plans (Medicare Plus Blue℠ PPO, BCN Advantage℠ HMO, BCN Advantage℠ HMO-POS) require prior authorization for certain benefits. Blue Cross and BCN recently launched the Medicare Advantage Prior Authorization webpage on bcbsm.com where you can quickly find clinical review criteria associated with services that require prior authorization. This new webpage puts the information you need in one convenient location. Reminder Before rendering services, make sure you check benefits, eligibility and medical policy coverage guidelines, using the self-service tools on our provider portal at availity.com.** **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Additional drugs to have a site-of-care requirement for some commercial members, starting Aug. 1For dates of service on or after Aug. 1, 2024, the following medical benefit drugs will have a site-of-care requirement for some Blue Cross Blue Shield of Michigan and all Blue Care Network group and individual commercial members:
When the site-of-care requirement goes into effect, these drugs may be covered only when administered at the following sites of care:
These drugs already require prior authorization through the Oncology Value Management program, administered by Carelon Medical Benefits Management. The new site-of-care requirement is in addition to any current prior authorization requirements. Commercial members affected by this change
Note: This requirement doesn’t apply to members who have coverage through the Blue Cross and Blue Shield Federal Employee Program®. How the site-of-care requirement will be phased in The site-of-care requirement will apply as follows for infusions involving the drugs listed above:
What to do for members who currently receive these drugs For Blue Cross and BCN commercial members who currently receive these drugs at an outpatient hospital facility:
For Blue Cross and BCN commercial members who currently receive these drugs at a provider’s office, at home or in an ambulatory infusion center, no action is required. How we’ll help For members who need to transition to a new infusion location, we’ll work with you and the member to facilitate the transition. We’ll notify members and encourage them to talk to you before changing their infusion location. We’ll also let them know that the change of location doesn’t affect the treatment you’re providing. List of requirements
Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services.
Amtagvi has additional requirements for most commercial membersBlue Cross Blue Shield of Michigan and Blue Care Network updated the medical policy for Amtagvi™ (lifileucel). The requirements in the updated medical policy apply for most Blue Cross and BCN commercial members for dates of service on or after May 28, 2024. The following additional requirements must be met for treatment with Amtagvi to be considered medically necessary:
You can see the full list of requirements in the updated medical policy. To view the policy, go to the Medical Policy Router Search page, enter the name of the drug in the Policy/Topic Keyword field and press Enter. To access the Medical Policy Router Search page, go to bcbsm.com/providers, click Resources and then click Search Medical Policies. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, these requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. Additional information For additional information about drugs covered under the medical benefit, see the following pages of the ereferrals.bcbsm.com website: Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
We’re granting a 90-day extension to the time limit for commercial claim submissionRecognizing that many providers were affected by the recent cyberattack on Change Healthcare, Blue Cross Blue Shield of Michigan and Blue Care Network are granting a 90‑day extension to the claim submission time limits for Blue Cross and BCN commercial claims. This includes primary original claims submitted on or after Feb. 22, 2024. The 90-day extension ends Sept. 30, 2024. For all primary original claims submitted on or after Oct. 1, 2024, existing participation or affiliation agreement submission deadlines will apply. The 90-day claim submission extension applies only to claims for Blue Cross commercial and BCN commercial. It doesn’t apply to Medicare Advantage (Medicare Plus Blue℠ or BCN Advantage℠), Medicare Supplement or other secondary claims. All audit rights and other plan rules still apply.
Clinical editing policies updatedIn support of appropriate coding and payment accuracy, we are providing the information below to keep you informed about payment policy updates, new policies and coding reminders. Blue Cross Blue Shield of Michigan commercial Anterior nasal hemorrhage and evaluation and management, or E/M, services Modifier 25 will no longer override claim editing for E/M services when billed with CPT code *30901 (control nasal hemorrhage, anterior, simple [limited cautery and/or packing] any method). The National Correct Coding Initiative, or NCCI, has established incidental code pairs between the E/M and minor procedure for control of nasal bleeding. Health care providers may receive a denial of E/M services when billed with *30901 beginning in September 2024. Blue Care Network commercial Q0091 HCPCS code Q0091 isn’t payable when reported with an E/M service. Providers are encouraged not to submit appeals for Q0091 as it is always included in the E/M service performed. Providers may see these denials as of April 2024. QVJ denials We previously published an alert informing providers of some claims denying in error. They were denied with code QVJ (not a covered service). These claims have since been adjusted for payment and this was completed in April 2024. BCN Advantage℠ Acupuncture CPT codes *20560 and *20561 will no longer deny when reported with *97810, *97811, *97813 or *97814. As of the end of April 2024, CPT codes *97810, *97811, *97813 or *97814 will deny and *20560 and *20561 will be the payable codes. Incorrect denials We previously informed you in February that some claims for preventive services and hearing services were incorrectly denied. They received a QN6 denial (not a covered service). These claims have been adjusted for payment. These adjustments were completed at the beginning of April 2024. Blue Care Network commercial and BCN Advantage G2212 The daily allowed units for G2212 have been updated to 6. This change aligns with Centers for Medicare & Medicaid Services guidelines and was effective in April 2024.
Omvoh SC and IV to have step therapy requirement for most commercial membersFor dates of service on or after June 3, 2024, members must try and fail four preferred products before we’ll approve prior authorization requests for Omvoh™ SC and IV (mirikizumab-mrkz), HCPCS code J3590. The four preferred products for Omvoh SC and IV are:
For the preferred products, health care providers will need to comply with any requirements, such as prior authorization, that apply under the applicable benefit. For Omvoh SC and IV:
We’ll update the Blue Cross and BCN utilization management medical drug list to reflect the preferred drugs. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, these requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. Additional information For more information about medical benefit drugs, see the following pages on ereferrals.bcbsm.com: Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
Select Medicare Advantage members will receive Cologuard test kit in JuneBlue Cross Blue Shield of Michigan and Blue Care Network are working with Exact Sciences, an existing, credentialed colorectal cancer screening provider, to distribute in-home Cologuard® test kits in June. The kits will go to select Medicare Plus Blue℠ PPO and BCN Advantage℠ members. Health care providers with patients who receive an advance notice letter about the kit should encourage them to take advantage of this convenient, no-cost screening. Members who have a gap in care for colorectal cancer screening will receive a Cologuard screening kit. Once completed, members will be encouraged to discuss test results with their primary care providers. Test result notification
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Cologuard by Exact Sciences is an independent company that provides colorectal testing services to Blue Cross Blue Shield of Michigan and Blue Care Network members.
Cosentyx IV to have a site-of-care requirement for most commercial members, starting July 1For dates of service on or after July 1, 2024, we’re adding a site-of-care requirement for Blue Cross Blue Shield of Michigan and Blue Care Network group and individual commercial members for the following drug covered under the medical benefit:
The NovoLogix® online tool will prompt you to select a site of care when you submit prior authorization requests for this drug. If the request meets the clinical criteria for the drug and is for one of the following sites of care, it will be approved automatically:
Additional information or documentation may be required for requests to administer Cosentyx IV in an outpatient hospital setting. As a reminder, this drug already requires prior authorization; providers can submit prior authorization requests using NovoLogix. The new site-of-care requirement is in addition to the current prior authorization requirement. Members who start courses of treatment with Cosentyx IV before July 1, 2024, will be able to continue receiving the drug in their current locations until their existing authorizations expire. If these members then continue treatment under new prior authorizations, the site-of-care requirement outlined above will apply. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, prior authorization and site-of-care requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. List of requirements For a full list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross commercial and BCN commercial members. We’ll update this list prior to the effective date. You can access this list and other information about requesting prior authorization at ereferrals.bcbsm.com, at these locations: Prior authorization isn't a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
Pemfexy and Pemrydi RTU to have additional step therapy requirements for most membersMembers must try and fail two other pemetrexed drugs before we’ll approve prior authorization requests for Pemfexy® or Pemrydi RTU®. For the details, refer to this table:
The preferred products are:
These drugs are covered under members’ medical benefits, not their pharmacy benefits. All of the drugs listed above continue to require prior authorization through the Carelon provider portal, as specified in the pertinent drug lists linked below. We’ll update these lists to reflect the new step therapy requirement prior to the effective date. Members affected by this change This requirement applies to the following members:
More about the prior authorization requirements For additional information on requirements related to drugs covered under the medical benefit, refer to the following drug lists:
As a reminder, prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members. *Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
Spevigo SC now has requirements for most commercial membersFor dates of service on or after April 25, 2024, we’ve added prior authorization and site-of-care requirements for most Blue Cross Blue Shield of Michigan and Blue Care Network group and individual commercial members for the following drug covered under the medical benefit:
How to submit prior authorization requests Submit prior authorization requests through the NovoLogix® online tool. It offers real-time status checks and immediate approvals for certain medications. To access NovoLogix, log in to our provider portal at availity.com,** click Payer Spaces in the menu bar, and then click the BCBSM and BCN logo. You’ll find links to the NovoLogix tools on the Applications tab. Note: If you need to request access to our provider portal, see the Register for web tools webpage on bcbsm.com. The NovoLogix online tool will prompt you to select a site of care when you submit prior authorization requests for this drug. If the request meets clinical criteria for the drug and is for one of the following sites of care, it will be approved automatically:
Additional information or documentation may be required for requests to administer Spevigo in an outpatient hospital setting. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, these requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group List. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. List of requirements For a full list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross commercial and BCN commercial members. You can access this list and other information about requesting prior authorization on the following pages of the ereferrals.bcbsm.com website: Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Change coming to nonclinical, transitional care program through Home & Community CareCurrently, the nonclinical, transitional care program through Home & Community Care (formerly known as naviHealth, Inc.) is available to Medicare Plus Blue℠ and BCN Advantage℠ members who are discharged to their homes or certain post-acute care facilities in Michigan from acute inpatient facilities. This program aims to reduce avoidable inpatient readmissions. On May 31, 2024, Home & Community Care will discontinue this 30-day program for members who are discharged to their homes. As a result, Home & Community Care navigation specialists won’t contact members discharged after May 1. This will ensure all members engaged in the program complete it by May 31. Starting June 1, 2024, the program will be available only to our Medicare Advantage members discharged to certain post-acute care facilities in Michigan. For more information about the program and to view the list of post-acute care facilities, see the document Nonclinical, transitional care program for Medicare Advantage. We’ll update our communications, including the document linked above, by June 1 to reflect this change. Home & Community Care is an independent company that provides nonclinical, transitional care services for Blue Cross Blue Shield of Michigan and Blue Care Network members who have Medicare Advantage plans.
TurningPoint opens peer-to-peer reviews to advanced practice providers for musculoskeletal, pain management proceduresThe TurningPoint Healthcare Solutions LLC is now scheduling peer-to-peer reviews with advanced practice providers, or APPs (physician assistants and nurse practitioners). The APP peer-to-peer review process is available for participating orthopedic, pain management and spinal surgical practices that are contracted with Blue Cross Blue Shield of Michigan, Blue Care Network or both. TurningPoint made this change to enable APPs to support physicians in the peer-to-peer review process. APPs can participate in peer-to-peer reviews related to routine prior authorization denials specific to coding, medical policy and documentation requirements for knee, ankle, shoulder, hip, elbow, wrist, spine and pain management procedures. Reviews will be conducted by providers of the same provider type. For example, if the requesting provider is a physician assistant, the review discussion will be scheduled with a physician assistant at TurningPoint. If you have questions about which cases are eligible for APP peer-to-peer reviews, contact the TurningPoint Provider Relations team at providersupport@turningpoint-healthcare.com. We recently posted the following TurningPoint documents to the Musculoskeletal Services and Pain Management Services pages on ereferrals.bcbsm.com. These documents are also available through the TurningPoint Provider Portal.
We’re updating the Musculoskeletal procedure authorizations: Frequently asked questions for providers document to reflect this change. Note: Provider offices will continue to have access to specialty-matched physician-to-physician peer-to-peer reviews. For more information about TurningPoint’s Musculoskeletal Surgical Quality and Safety Management program, including information about which groups and members participate in the program, see the following pages on ereferrals.bcbsm.com:
TurningPoint Healthcare Solutions LLC is an independent company provides care review services for Blue Cross Blue Shield of Michigan and Blue Care Network.
Register now for 2024 virtual provider symposium sessionsThe last sessions of the virtual provider symposiums focusing on quality measures, documentation, and coding guidelines are at the beginning of June. Registration is open on the provider training website. Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending. Once you’re logged in to the provider training site, open the event calendar to sign up for any of the sessions listed below. You can also quickly search for all the sessions with the keyword “symposium” by looking under the results for Events. All Star Performance-HEDIS® / Star Rating Measure Overview: For physicians and office staff responsible for closing gaps in care related to quality adult measures
Coding and Documentation Tips for 2024 and Beyond: For physicians, coders, billers and administrative staff
Provider training website access You can also directly access the training website if you don’t have a provider portal account. Questions?
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. HEDIS® is a registered trademark of the National Committee for Quality Assurance. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services. Accreditation Statement: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the Minnesota Medical Association and BCBS of Michigan. The Minnesota Medical Association (MMA) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. CME Statement: The Minnesota Medical Association designates this internet live activity for a maximum of 2 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Vyjuvek now has site-of-care requirement for most commercial membersFor dates of service on or after April 4, 2024, we’ve added a site-of-care requirement for Blue Cross Blue Shield of Michigan and Blue Care Network group and individual commercial members for the following drug covered under the medical benefit:
The NovoLogix® online tool will prompt you to select a site of care when you submit prior authorization requests for this drug. If the request meets the clinical criteria for the drug and is for one of the following sites of care, it will be approved automatically:
Additional information or documentation may be required for requests to administer Vyjuvek in an outpatient hospital setting. This drug already requires prior authorization; providers can submit prior authorization requests using NovoLogix. The new site-of-care requirement is in addition to the current prior authorization requirement. Members who started courses of treatment with Vyjuvek before April 4, 2024, will be able to continue receiving the drug in their current location until their existing authorization expires. If these members then continue treatment under a new prior authorization, the site-of-care requirement outlined above will apply. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial groups, prior authorization and site-of-care requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. List of requirements For a full list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross commercial and BCN commercial members. Prior authorization isn't a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
Save time by submitting only required information about acute inpatient medical, surgical admissionsWhen health care providers submit a prior authorization request for an acute inpatient medical-surgical admission, they can save time by submitting only the information that’s required for the request. Refer to the table below for more information.
TurningPoint Healthcare Solutions LLC is an independent company that manages authorizations for musculoskeletal surgical and related procedures for Blue Cross Blue Shield of Michigan and Blue Care Network.
AllianceRx Walgreens to become exclusive specialty drug pharmacy for MESSA, starting July 1, 2024AllianceRx Walgreens Pharmacy will become the exclusive specialty drug network for the Michigan Education Special Services Association, known as MESSA, starting July 1, 2024. MESSA members who take a specialty drug have been directed to transfer their specialty medication to AllianceRx Walgreens. If you have patients who don’t currently fill their specialty drugs with AllianceRx Walgreens, you’ll need to transfer their prescriptions to AllianceRx Walgreens before July 1, 2024. If MESSA members fill their specialty medication through a different pharmacy, beginning July 1, 2024, they may be responsible for the full cost of the drug. Note: On Aug. 1, 2024, AllianceRx Walgreens Pharmacy will change its name to Walgreens Specialty Pharmacy.
Guidelines for using Autism diagnostic evaluation results formMembers can obtain an autism evaluation using one of the methods described in the document Obtaining an autism diagnostic evaluation and finding treatment. If you choose to record the results of the autism evaluation on the Autism diagnostic evaluation results form, follow these guidelines:
Note: We’re still receiving the older forms from approved autism evaluation centers. Faxing these forms to Blue Cross Blue Shield of Michigan and Blue Care Network — instead of giving them to the member or to the member’s parent or guardian — can delay the members from getting the treatment they need.
We’ve updated the document Blue Cross Behavioral Health: Frequently asked questions for providers to include this information. Providers can access that document on our ereferrals.bcbsm.com website, on these pages:
Webinars for physicians, coders focus on risk adjustment, codingWe’re offering webinars about documentation and coding of common challenging diagnoses. These live, lunchtime educational sessions will also include an opportunity to ask questions. Below is our schedule and the tentative topics for the sessions. All sessions start at noon Eastern time and generally last for 30 minutes. Register for the session that best works with your schedule on the provider training website.
Provider training website access Provider portal users with an Availity® Essentials account can access the provider training website by logging in to availity.com,** clicking on Payer Space in the top menu bar and then clicking on the BCBSM and BCN logo. Then click on the Applications tab, scroll down to the Provider Training Site tile and click on it. You can also directly access the training website if you don’t have a provider portal account: Provider training website. After logging in to the provider training website, look in Event Calendar to sign up for your desired session. You can also quickly search for all the sessions with the keyword “lunchtime" and then look under the results for Events. You can listen to the previously recorded sessions too. Check out the following:
Questions?
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Here’s what you need to know about the proposed ambulance purchased services program and how to provide feedbackBlue Cross Blue Shield of Michigan is proposing the implementation of an ambulance purchased services program. This program will include ambulance services from the originating facility to another facility and then return to the originating facility in the purchased services definition. Program implementation is scheduled for fourth quarter 2024, for Blue Cross commercial and Blue Care Network commercial. Ambulance transports will be added to the purchased services definition found in the Participating Hospital Agreement Payment Manual.
What this means to facilities Facilities reimbursed by DRG or per diem will be responsible for the cost of any ambulance transport from their originating hospital to another facility and then returning to their originating facility. Example: Patient is admitted to facility A and needs an open MRI, but facility A doesn't have the equipment. Patient is transported by ambulance to facility B for the procedure. The patient is then transported back to facility A by ambulance. Facility A will be responsible for the transport costs in addition to the purchased services. Ambulance services will be subject to the PHA Payment Manual’s "Purchased Services" section 5.4: Only the hospital with the inpatient admission may bill for services provided to that patient during the inpatient stay. Payment for purchased services is as follows:
Input requested According to the Contract Administration Process — part of the Participating Hospital Agreement that went into effect July 1, 2021 — we allow non-binding input from participating facilities about such proposals. Input from facilities is requested by June 30, 2024. Send any input you may have to Lauren Rossi at LRossi2@bcbsm.com. After input is received, Blue Cross has 30 calendar days to respond to input.
We’ve enhanced the Authorizations & Referrals tool in Availity EssentialsIn May 2024, we made an enhancement to the Authorizations & Referrals tool in our provider portal, Availity® Essentials. This enhancement makes it easier for health care providers to determine if a Carelon-managed procedure code requires prior authorization for an in-state or out-of-state Blue Cross Blue Shield of Michigan commercial member. Now, when you use the Authorizations & Referrals tool to look up the prior authorization requirement for a Carelon-managed procedure code, you’ll be directed to Carelon if prior authorization is required for the service for that specific member. If prior authorization isn’t required, you’ll see a message to that effect. To access the Authorizations & Referrals tool:
For instructions on how to use the Authorizations & Referrals tool, see the document titled Determining prior authorization requirements for members. You can also access this document by clicking the Determine prior authorization requirements for members tile on the left side of any page on our ereferrals.bcbsm.com website. **Blue Cross Blue Shield of Michigan doesn’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services. Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
No application necessary for certain specialties to join BlueHPNThe Blue High Performance Network℠, also called BlueHPN℠, no longer requires the following specialties in the Detroit market to apply for the network to keep their active status:
This is in addition to behavioral health specialties, which we reported in the January Record require no application to keep their active status. The effective date of participation all the specialties listed above went into effect Jan. 1, 2024. To support adequate access to the specialties listed above, Blue Cross Blue Shield of Michigan will use the TRUST PPO or Traditional network to support BlueHPN to ensure inclusivity to members who need these services. By removing the application process for these specialties, it allows BlueHPN members to choose from a broader range of in-network providers for these services, while increasing access to care. Providers who are part of the BlueHPN must at a minimum:
If you have questions, email the BlueHPN team at BlueHPN@bcbsm.com.
Change to prior authorization process for Blue Cross commercial members in Michigan whose plans have local provider networksBlue Cross Blue Shield of Michigan currently has one Blue Cross commercial plan in Michigan that has a local provider network. That plan is the Blue High Performance Network℠, or BlueHPN℠. Because this plan has a local provider network, providers must be in that network for services to be reimbursable. Starting June 27, 2024, we’ll update the prior authorization process for members with BlueHPN plans to ensure that providers are in network. We’ll also do this for any Blue Cross commercial plans with local provider networks that are added in the future for members in Michigan. Here’s what we’ll do on and after June 27 when we receive prior authorization requests for members with these plans:
Reminders As always, it’s essential that providers check each member’s eligibility and benefits prior to performing services. Providers are responsible for identifying the need for prior authorization through our provider portal, Benefit Explainer or Provider Inquiry and for obtaining prior authorization for services, as needed.
Additional drugs to have a site-of-care requirement for some commercial members, starting Aug. 1For dates of service on or after Aug. 1, 2024, the following medical benefit drugs will have a site-of-care requirement for some Blue Cross Blue Shield of Michigan and all Blue Care Network group and individual commercial members:
When the site-of-care requirement goes into effect, these drugs may be covered only when administered at the following sites of care:
These drugs already require prior authorization through the Oncology Value Management program, administered by Carelon Medical Benefits Management. The new site-of-care requirement is in addition to any current prior authorization requirements. Commercial members affected by this change
Note: This requirement doesn’t apply to members who have coverage through the Blue Cross and Blue Shield Federal Employee Program®. How the site-of-care requirement will be phased in The site-of-care requirement will apply as follows for infusions involving the drugs listed above:
What to do for members who currently receive these drugs For Blue Cross and BCN commercial members who currently receive these drugs at an outpatient hospital facility:
For Blue Cross and BCN commercial members who currently receive these drugs at a provider’s office, at home or in an ambulatory infusion center, no action is required. How we’ll help For members who need to transition to a new infusion location, we’ll work with you and the member to facilitate the transition. We’ll notify members and encourage them to talk to you before changing their infusion location. We’ll also let them know that the change of location doesn’t affect the treatment you’re providing. List of requirements
Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage authorizations for select services.
Amtagvi has additional requirements for most commercial membersBlue Cross Blue Shield of Michigan and Blue Care Network updated the medical policy for Amtagvi™ (lifileucel). The requirements in the updated medical policy apply for most Blue Cross and BCN commercial members for dates of service on or after May 28, 2024. The following additional requirements must be met for treatment with Amtagvi to be considered medically necessary:
You can see the full list of requirements in the updated medical policy. To view the policy, go to the Medical Policy Router Search page, enter the name of the drug in the Policy/Topic Keyword field and press Enter. To access the Medical Policy Router Search page, go to bcbsm.com/providers, click Resources and then click Search Medical Policies. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, these requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. Additional information For additional information about drugs covered under the medical benefit, see the following pages of the ereferrals.bcbsm.com website: Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
We’re granting a 90-day extension to the time limit for commercial claim submissionRecognizing that many providers were affected by the recent cyberattack on Change Healthcare, Blue Cross Blue Shield of Michigan and Blue Care Network are granting a 90‑day extension to the claim submission time limits for Blue Cross and BCN commercial claims. This includes primary original claims submitted on or after Feb. 22, 2024. The 90-day extension ends Sept. 30, 2024. For all primary original claims submitted on or after Oct. 1, 2024, existing participation or affiliation agreement submission deadlines will apply. The 90-day claim submission extension applies only to claims for Blue Cross commercial and BCN commercial. It doesn’t apply to Medicare Advantage (Medicare Plus Blue℠ or BCN Advantage℠), Medicare Supplement or other secondary claims. All audit rights and other plan rules still apply.
Clinical editing policies updatedIn support of appropriate coding and payment accuracy, we are providing the information below to keep you informed about payment policy updates, new policies and coding reminders. Blue Cross Blue Shield of Michigan commercial Anterior nasal hemorrhage and evaluation and management, or E/M, services Modifier 25 will no longer override claim editing for E/M services when billed with CPT code *30901 (control nasal hemorrhage, anterior, simple [limited cautery and/or packing] any method). The National Correct Coding Initiative, or NCCI, has established incidental code pairs between the E/M and minor procedure for control of nasal bleeding. Health care providers may receive a denial of E/M services when billed with *30901 beginning in September 2024. Blue Care Network commercial Q0091 HCPCS code Q0091 isn’t payable when reported with an E/M service. Providers are encouraged not to submit appeals for Q0091 as it is always included in the E/M service performed. Providers may see these denials as of April 2024. QVJ denials We previously published an alert informing providers of some claims denying in error. They were denied with code QVJ (not a covered service). These claims have since been adjusted for payment and this was completed in April 2024. BCN Advantage℠ Acupuncture CPT codes *20560 and *20561 will no longer deny when reported with *97810, *97811, *97813 or *97814. As of the end of April 2024, CPT codes *97810, *97811, *97813 or *97814 will deny and *20560 and *20561 will be the payable codes. Incorrect denials We previously informed you in February that some claims for preventive services and hearing services were incorrectly denied. They received a QN6 denial (not a covered service). These claims have been adjusted for payment. These adjustments were completed at the beginning of April 2024. Blue Care Network commercial and BCN Advantage G2212 The daily allowed units for G2212 have been updated to 6. This change aligns with Centers for Medicare & Medicaid Services guidelines and was effective in April 2024.
Vendor change for commercial DRG audits beginning June 2024Blue Cross Blue Shield of Michigan previously worked with Change Healthcare to perform commercial diagnosis-related group, or DRG, validation audits as part of the Payment Integrity Program. Change Healthcare has been acquired by Optum. Optum, an independent company, will provide auditing support for Blue Cross by performing claim audits on inpatient hospital DRG claims beginning in June 2024. The audits will:
Medical records will be reviewed to:
You’ll need to provide medical records for review at the time of an audit. After an audit, Optum will send you a determination letter that details information on how to request an appeal. If you have questions, contact an Optum provider service representative at 1-877-787-2310 from 8 a.m. to 4 p.m. Eastern time Monday through Friday.
Emergency Department Claim Analyzer reminder and reconsideration programAs a reminder, the Emergency Department Claim Analyze, or EDCA, program was re-implemented on June 1, 2024, in conjunction with a new Emergency Department Facility Evaluation and Management reimbursement policy. This reimbursement policy was developed to ensure that facilities are reimbursed based on the consistent coding that correctly describes the patient’s clinical condition and the health care services provided in accordance with industry standards and Centers for Medicare & Medicaid Services guidelines. What does this mean to hospitals? Blue Cross is reviewing evaluation and management, or E/M, claims that are billed with a level four or five E/M code (*99284 or *99285) for the appropriate level of care on a prepayment basis. Claims that don't meet the policy criteria will be adjusted and reimbursed at the appropriate level. At this time, claims that are two or more levels higher than the Blue Cross reimbursement policy will be adjusted. Claims that are one level higher won't be adjusted. Blue Cross will continue to monitor all emergency department claims submitted. We reserve the right to modify the scope if adherence and adjustments don't align with the reimbursement policy. Hospitals reimbursed by the fee schedule For hospitals reimbursed via the fee schedule, claims recommended for adjustment will be adjusted to the appropriate level. These claims will be paid according to the fee schedule requiring no additional action by the hospitals. The change will be reflected in the provider voucher and 835 electronic transaction. This will also be shown with a CO186 adjustment code, level of care change adjustment. Hospitals reimbursed by percent of charge For hospitals reimbursed percent of charge, emergency department claims recommended for adjustment will be denied. Remittance will be provided with information on how to be reimbursed and will indicate the appropriate level recommended for approval. It will require hospitals to adjust the claim and charges associated and re-submit to Blue Cross for processing and payment. What should I do if I am a percent of charge hospital, and my claim was denied? You can submit a corrected claim through either:
What should I do if I disagree with the claim adjustment? Providers who disagree with the claim adjustment have the right to submit a reconsideration request to have their claim reviewed. To have the claim reviewed, providers must supply:
If these items aren't provided, the claim will remain adjusted/denied and the reconsideration will be upheld. To submit a reconsideration using Availity, please complete the following steps:
Note: If you want to initiate appeals on additional claims, click Close to return to the Claim Status results page. To continue your appeal of the claim in question, complete the additional steps outlined here.  Important: In the field shown below, enter this information: 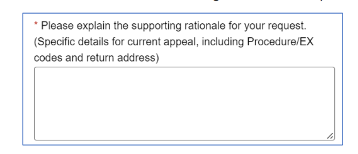 The Emergency Department Facility Evaluation and Management Reimbursement Policy can be found on the Provider Resources website:
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website.
Omvoh SC and IV to have step therapy requirement for most commercial membersFor dates of service on or after June 3, 2024, members must try and fail four preferred products before we’ll approve prior authorization requests for Omvoh™ SC and IV (mirikizumab-mrkz), HCPCS code J3590. The four preferred products for Omvoh SC and IV are:
For the preferred products, health care providers will need to comply with any requirements, such as prior authorization, that apply under the applicable benefit. For Omvoh SC and IV:
We’ll update the Blue Cross and BCN utilization management medical drug list to reflect the preferred drugs. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, these requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. Additional information For more information about medical benefit drugs, see the following pages on ereferrals.bcbsm.com: Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
Select Medicare Advantage members will receive Cologuard test kit in JuneBlue Cross Blue Shield of Michigan and Blue Care Network are working with Exact Sciences, an existing, credentialed colorectal cancer screening provider, to distribute in-home Cologuard® test kits in June. The kits will go to select Medicare Plus Blue℠ PPO and BCN Advantage℠ members. Health care providers with patients who receive an advance notice letter about the kit should encourage them to take advantage of this convenient, no-cost screening. Members who have a gap in care for colorectal cancer screening will receive a Cologuard screening kit. Once completed, members will be encouraged to discuss test results with their primary care providers. Test result notification
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Cologuard by Exact Sciences is an independent company that provides colorectal testing services to Blue Cross Blue Shield of Michigan and Blue Care Network members.
Cosentyx IV to have a site-of-care requirement for most commercial members, starting July 1For dates of service on or after July 1, 2024, we’re adding a site-of-care requirement for Blue Cross Blue Shield of Michigan and Blue Care Network group and individual commercial members for the following drug covered under the medical benefit:
The NovoLogix® online tool will prompt you to select a site of care when you submit prior authorization requests for this drug. If the request meets the clinical criteria for the drug and is for one of the following sites of care, it will be approved automatically:
Additional information or documentation may be required for requests to administer Cosentyx IV in an outpatient hospital setting. As a reminder, this drug already requires prior authorization; providers can submit prior authorization requests using NovoLogix. The new site-of-care requirement is in addition to the current prior authorization requirement. Members who start courses of treatment with Cosentyx IV before July 1, 2024, will be able to continue receiving the drug in their current locations until their existing authorizations expire. If these members then continue treatment under new prior authorizations, the site-of-care requirement outlined above will apply. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, prior authorization and site-of-care requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. List of requirements For a full list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross commercial and BCN commercial members. We’ll update this list prior to the effective date. You can access this list and other information about requesting prior authorization at ereferrals.bcbsm.com, at these locations: Prior authorization isn't a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members.
Pemfexy and Pemrydi RTU to have additional step therapy requirements for most membersMembers must try and fail two other pemetrexed drugs before we’ll approve prior authorization requests for Pemfexy® or Pemrydi RTU®. For the details, refer to this table:
The preferred products are:
These drugs are covered under members’ medical benefits, not their pharmacy benefits. All of the drugs listed above continue to require prior authorization through the Carelon provider portal, as specified in the pertinent drug lists linked below. We’ll update these lists to reflect the new step therapy requirement prior to the effective date. Members affected by this change This requirement applies to the following members:
More about the prior authorization requirements For additional information on requirements related to drugs covered under the medical benefit, refer to the following drug lists:
As a reminder, prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members. *Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Carelon Medical Benefits Management is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to manage prior authorizations for select services.
Spevigo SC now has requirements for most commercial membersFor dates of service on or after April 25, 2024, we’ve added prior authorization and site-of-care requirements for most Blue Cross Blue Shield of Michigan and Blue Care Network group and individual commercial members for the following drug covered under the medical benefit:
How to submit prior authorization requests Submit prior authorization requests through the NovoLogix® online tool. It offers real-time status checks and immediate approvals for certain medications. To access NovoLogix, log in to our provider portal at availity.com,** click Payer Spaces in the menu bar, and then click the BCBSM and BCN logo. You’ll find links to the NovoLogix tools on the Applications tab. Note: If you need to request access to our provider portal, see the Register for web tools webpage on bcbsm.com. The NovoLogix online tool will prompt you to select a site of care when you submit prior authorization requests for this drug. If the request meets clinical criteria for the drug and is for one of the following sites of care, it will be approved automatically:
Additional information or documentation may be required for requests to administer Spevigo in an outpatient hospital setting. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial, these requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group List. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. List of requirements For a full list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross commercial and BCN commercial members. You can access this list and other information about requesting prior authorization on the following pages of the ereferrals.bcbsm.com website: Prior authorization isn’t a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members. **Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services.
Change coming to nonclinical, transitional care program through Home & Community CareCurrently, the nonclinical, transitional care program through Home & Community Care (formerly known as naviHealth, Inc.) is available to Medicare Plus Blue℠ and BCN Advantage℠ members who are discharged to their homes or certain post-acute care facilities in Michigan from acute inpatient facilities. This program aims to reduce avoidable inpatient readmissions. On May 31, 2024, Home & Community Care will discontinue this 30-day program for members who are discharged to their homes. As a result, Home & Community Care navigation specialists won’t contact members discharged after May 1. This will ensure all members engaged in the program complete it by May 31. Starting June 1, 2024, the program will be available only to our Medicare Advantage members discharged to certain post-acute care facilities in Michigan. For more information about the program and to view the list of post-acute care facilities, see the document Nonclinical, transitional care program for Medicare Advantage. We’ll update our communications, including the document linked above, by June 1 to reflect this change. Home & Community Care is an independent company that provides nonclinical, transitional care services for Blue Cross Blue Shield of Michigan and Blue Care Network members who have Medicare Advantage plans.
TurningPoint opens peer-to-peer reviews to advanced practice providers for musculoskeletal, pain management proceduresThe TurningPoint Healthcare Solutions LLC is now scheduling peer-to-peer reviews with advanced practice providers, or APPs (physician assistants and nurse practitioners). The APP peer-to-peer review process is available for participating orthopedic, pain management and spinal surgical practices that are contracted with Blue Cross Blue Shield of Michigan, Blue Care Network or both. TurningPoint made this change to enable APPs to support physicians in the peer-to-peer review process. APPs can participate in peer-to-peer reviews related to routine prior authorization denials specific to coding, medical policy and documentation requirements for knee, ankle, shoulder, hip, elbow, wrist, spine and pain management procedures. Reviews will be conducted by providers of the same provider type. For example, if the requesting provider is a physician assistant, the review discussion will be scheduled with a physician assistant at TurningPoint. If you have questions about which cases are eligible for APP peer-to-peer reviews, contact the TurningPoint Provider Relations team at providersupport@turningpoint-healthcare.com. We recently posted the following TurningPoint documents to the Musculoskeletal Services and Pain Management Services pages on ereferrals.bcbsm.com. These documents are also available through the TurningPoint Provider Portal.
We’re updating the Musculoskeletal procedure authorizations: Frequently asked questions for providers document to reflect this change. Note: Provider offices will continue to have access to specialty-matched physician-to-physician peer-to-peer reviews. For more information about TurningPoint’s Musculoskeletal Surgical Quality and Safety Management program, including information about which groups and members participate in the program, see the following pages on ereferrals.bcbsm.com:
TurningPoint Healthcare Solutions LLC is an independent company provides care review services for Blue Cross Blue Shield of Michigan and Blue Care Network.
Register now for 2024 virtual provider symposium sessionsThe last sessions of the virtual provider symposiums focusing on quality measures, documentation, and coding guidelines are at the beginning of June. Registration is open on the provider training website. Physicians, physician assistants, nurse practitioners, nurses and coders can receive continuing education credits for attending. Once you’re logged in to the provider training site, open the event calendar to sign up for any of the sessions listed below. You can also quickly search for all the sessions with the keyword “symposium” by looking under the results for Events. All Star Performance-HEDIS® / Star Rating Measure Overview: For physicians and office staff responsible for closing gaps in care related to quality adult measures
Coding and Documentation Tips for 2024 and Beyond: For physicians, coders, billers and administrative staff
Provider training website access You can also directly access the training website if you don’t have a provider portal account. Questions?
**Blue Cross Blue Shield of Michigan and Blue Care Network don’t own or control this website. HEDIS® is a registered trademark of the National Committee for Quality Assurance. Availity® is an independent company that contracts with Blue Cross Blue Shield of Michigan and Blue Care Network to offer provider portal and electronic data interchange services. Accreditation Statement: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the Minnesota Medical Association and BCBS of Michigan. The Minnesota Medical Association (MMA) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. CME Statement: The Minnesota Medical Association designates this internet live activity for a maximum of 2 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Vyjuvek now has site-of-care requirement for most commercial membersFor dates of service on or after April 4, 2024, we’ve added a site-of-care requirement for Blue Cross Blue Shield of Michigan and Blue Care Network group and individual commercial members for the following drug covered under the medical benefit:
The NovoLogix® online tool will prompt you to select a site of care when you submit prior authorization requests for this drug. If the request meets the clinical criteria for the drug and is for one of the following sites of care, it will be approved automatically:
Additional information or documentation may be required for requests to administer Vyjuvek in an outpatient hospital setting. This drug already requires prior authorization; providers can submit prior authorization requests using NovoLogix. The new site-of-care requirement is in addition to the current prior authorization requirement. Members who started courses of treatment with Vyjuvek before April 4, 2024, will be able to continue receiving the drug in their current location until their existing authorization expires. If these members then continue treatment under a new prior authorization, the site-of-care requirement outlined above will apply. Some Blue Cross commercial groups aren’t subject to these requirements For Blue Cross commercial groups, prior authorization and site-of-care requirements apply only to groups that participate in the standard commercial Medical Drug Prior Authorization Program for drugs administered under the medical benefit. To determine whether a group participates in the prior authorization program, see the Specialty Pharmacy Prior Authorization Master Opt-in/out Group list. Note: Blue Cross and Blue Shield Federal Employee Program® members and UAW Retiree Medical Benefits Trust (non-Medicare) members don’t participate in the standard prior authorization program. List of requirements For a full list of requirements related to drugs covered under the medical benefit, see the Blue Cross and BCN utilization management medical drug list for Blue Cross commercial and BCN commercial members. Prior authorization isn't a guarantee of payment. Health care practitioners need to verify eligibility and benefits for members. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
No portion of this publication may be copied without the express written permission of Blue Cross Blue Shield of Michigan, except that BCBSM participating health care providers may make copies for their personal use. In no event may any portion of this publication be copied or reprinted and used for commercial purposes by any party other than BCBSM.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||